You are using a browser that is not supported by this site. The site will not function properly. Please switch to the latest version of a supported browser such as Chrome, Safari, Edge, or Firefox to use this site.
Intra-page navigation
TITRATION SCHEDULING ACCORDING TO CLINICAL EXPERIENCE
Start your patient at a dose of 1.25 ng/kg/min. Titrate up in 1.25 ng/kg/min increments per week to achieve a dose that improves PAH symptoms while minimizing excessive pharmacologic effects of Remodulin (ie, headache, nausea, emesis, restlessness, anxiety, and infusion site pain or reaction).1
- In the pivotal, randomized, controlled studies of SC Remodulin, the mean dose at week 12 was 9.3 ng/kg/min1
- Dose adjustments can be made as frequently as tolerated1
| Initiate | First 4 Weeks | After Week 4 |
|---|---|---|
| Initiate |
Increase in |
Increase in |
Titrate slowly in patients with hepatic or renal insufficiency. These patients will likely be exposed to greater systemic concentrations than patients with normal hepatic or renal function.1 If inhibitors or inducers of CYP2C8 are added or withdrawn, you should also adjust treatment doses. Co-administration of Remodulin with a CYP2C8 inhibitor increases exposure to treprostinil and with an inducer decreases exposure to treprostinil.1
HEAR FROM A PAH EXPERT
Dosing with Remodulin
Robert Schilz, DO, explains the importance of optimized dosing with Remodulin while balancing the art and science of titrating to therapeutic effect.
Real-World Dosing Experience2
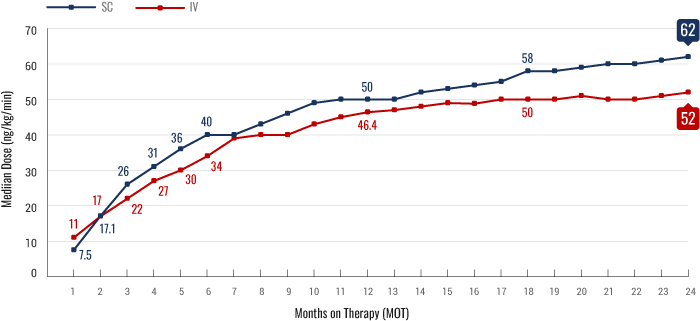
FIGURE. Median outpatient dosing of subcutaneous (SC) treprostinil compared to intravenous (IV) treprostinil at each month on therapy. Based on a retrospective analysis of 2647 patient medication shipment records from specialty pharmacy services between January 2009 and August 2018 (n=1040 for IV; n=1607 for SC). Dose titration was determined by calculating the dose acceleration rate (DAR), assessing the slope of dose increase between month on therapy 1 (MOT1) and the end of the time period assessed.
Real-world dosing should be interpreted with appropriate caution. Time points are subject to patient attrition or discontinuation from treatment
FIGURE. Median outpatient dosing of subcutaneous (SC) treprostinil compared to intravenous (IV) treprostinil at each month on therapy. Based on a retrospective analysis of 2647 patient medication shipment records from specialty pharmacy services between January 2009 and August 2018 (n=1040 for IV; n=1607 for SC). Dose titration was determined by calculating the dose acceleration rate (DAR), assessing the slope of dose increase between month on therapy 1 (MOT1) and the end of the time period assessed.
Average dose in real-world use of Remodulin3
Based on Specialty Pharmacy shipment data during January 2024. Dose data reported in Specialty Pharmacy shipments only. Limited to unique quarterly patients with dosing data.
Calculating SC (undiluted) Remodulin Infusion rate1:
INFUSION RATE (mL/hr)
*Conversion factor of 0.00006 = 60 min/hour x 0.000001 mg/ng.
Calculating Amount of IV (diluted) Remodulin Injection1:
Step 1: Calculate Diluted Remodulin Concentration (mg/mL)
Step 2: Calculate Amount of Remodulin Injection (mL)
The calculated volume of Remodulin Injection is then added to the reservoir along with the sufficient volume of diluent to achieve the desired total volume in the reservoir.
IV=intravenous; PAH=pulmonary arterial hypertension; SC=subcutaneous.
Important Safety Information
Warnings and Precautions
- Chronic intravenous (IV) infusions of Remodulin delivered using an external infusion pump with an indwelling central venous catheter are associated with the risk of blood stream infections (BSIs) and sepsis, which may be fatal. Therefore, continuous subcutaneous (SC) infusion is the preferred mode of administration.
- Avoid abrupt withdrawal or sudden large reductions in dosage of Remodulin, which may result in worsening of PAH symptoms.
- Titrate slowly in patients with hepatic or renal insufficiency, because such patients will likely be exposed to greater systemic concentrations relative to patients with normal hepatic or renal function.
- Remodulin is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Remodulin may produce symptomatic hypotension.
- Remodulin inhibits platelet aggregation and increases the risk of bleeding.
Adverse Reactions
- In clinical studies of SC Remodulin infusion, the most common adverse events reported were infusion site pain and infusion site reaction (redness, swelling, and rash). These symptoms were sometimes severe and sometimes required treatment with narcotics or discontinuation of Remodulin. The IV infusion of Remodulin with an external infusion pump has been associated with a risk of blood stream infections, arm swelling, paresthesias, hematoma, and pain. Other common adverse events (≥3% more than placebo) seen with either SC or IV Remodulin were headache (27% vs. 23%), diarrhea (25% vs. 16%), nausea (22% vs. 18%), rash (14% vs. 11%), jaw pain (13% vs. 5%), vasodilatation (11% vs. 5%), edema (9% vs. 3%), and hypotension (4% vs. 2%).
Drug Interactions
- Remodulin dosage adjustment may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn.
Specific Populations
- In patients with mild or moderate hepatic insufficiency, decrease the initial dose of Remodulin to 0.625 ng/kg/min of ideal body weight, and monitor closely. Remodulin has not been studied in patients with severe hepatic insufficiency.
- Safety and effectiveness of Remodulin in pediatric patients have not been established.
- It is unknown if geriatric patients respond differently than younger patients. Caution should be used when selecting a dose for geriatric patients.
- There are no adequate and well-controlled studies with Remodulin in pregnant women. It is not known whether treprostinil is excreted in human milk or if it affects the breastfed infant or milk production.
Indication
Remodulin is a prostacyclin vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%).
In patients with PAH requiring transition from epoprostenol, Remodulin is indicated to diminish the rate of clinical deterioration. Consider the risks and benefits of each drug prior to transition.
REMISIhcpMAY2021
Please see accompanying Full Prescribing Information for Remodulin.
For additional information, visit www.RemodulinPro.com or call Customer Service at 1-877-UNITHER (1-877-864-8437).
For additional information, visit www.RemodulinPro.com or call Customer Service at 1-877-UNITHER (1-877-864-8437).
Important Safety Information
Warnings and Precautions
- Chronic intravenous (IV) infusions of Remodulin delivered using an external infusion pump with an indwelling central venous catheter are associated with the risk of blood stream infections (BSIs) and sepsis, which may be fatal. Therefore, continuous subcutaneous (SC) infusion is the preferred mode of administration.
- Avoid abrupt withdrawal or sudden large reductions in dosage of Remodulin, which may result in worsening of PAH symptoms.
- Titrate slowly in patients with hepatic or renal insufficiency, because such patients will likely be exposed to greater systemic concentrations relative to patients with normal hepatic or renal function.
- Remodulin is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Remodulin may produce symptomatic hypotension.
- Remodulin inhibits platelet aggregation and increases the risk of bleeding.
Adverse Reactions
- In clinical studies of SC Remodulin infusion, the most common adverse events reported were infusion site pain and infusion site reaction (redness, swelling, and rash). These symptoms were sometimes severe and sometimes required treatment with narcotics or discontinuation of Remodulin. The IV infusion of Remodulin with an external infusion pump has been associated with a risk of blood stream infections, arm swelling, paresthesias, hematoma, and pain. Other common adverse events (≥3% more than placebo) seen with either SC or IV Remodulin were headache (27% vs. 23%), diarrhea (25% vs. 16%), nausea (22% vs. 18%), rash (14% vs. 11%), jaw pain (13% vs. 5%), vasodilatation (11% vs. 5%), edema (9% vs. 3%), and hypotension (4% vs. 2%).
Drug Interactions
- Remodulin dosage adjustment may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn.
Specific Populations
- In patients with mild or moderate hepatic insufficiency, decrease the initial dose of Remodulin to 0.625 ng/kg/min of ideal body weight, and monitor closely. Remodulin has not been studied in patients with severe hepatic insufficiency.
- Safety and effectiveness of Remodulin in pediatric patients have not been established.
- It is unknown if geriatric patients respond differently than younger patients. Caution should be used when selecting a dose for geriatric patients.
- There are no adequate and well-controlled studies with Remodulin in pregnant women. It is not known whether treprostinil is excreted in human milk or if it affects the breastfed infant or milk production.
Indication
Remodulin is a prostacyclin vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%).
In patients with PAH requiring transition from epoprostenol, Remodulin is indicated to diminish the rate of clinical deterioration. Consider the risks and benefits of each drug prior to transition.
REMISIhcpMAY2021
Please see accompanying Full Prescribing Information for Remodulin.
For additional information, visit www.RemodulinPro.com or call Customer Service at 1-877-UNITHER (1-877-864-8437).
References: 1. Remodulin [package insert]. Research Triangle Park, NC: United Therapeutics Corporation; 2023. 2. Balasubramanian VP, Safdar Z, Sketch MR, et al. Real-world dosing characteristics and utilization of parenteral treprostinil in the outpatient setting. Pulm Circ. 2022;12(1):e12016. Published 2022 Jan 12. doi:10.1002/pul2.12016 3. Data on file. United Therapeutics Corporation. Research Triangle Park, NC.
